ARC-DISCHARGE-SUNLAMPS
In a mercury vapour arc discharge sunlamp, a voltaic arc was established between
two electrodes that were located at the outer ends of a sealed glass tube with a
DC-voltage source applied over the electrodes. The electrode carrying the positive
voltage was called the anode while the other one was called the cathode. The
potential difference caused an electric field between the electrodes and this field
issued a force that attracted electrons in the cathode towards the anode. In case of a
sufficiently strong field it could occur that electrons were pulled out from the cathode
and when there was a vacuum within the tube, these electrons would travel towards
the anode at an ever-increasing speed. Their trip ended when they collided with the
anode and recombined with one of its atoms, releasing their energy with the radiation
of a photon. Such a collision did not provide the ultraviolet radiation that was
required for a sunlamp however. To create an effective sunlamp, the tube had to be
filled with a gas, causing collisions between the gas atoms and the electrons that
emerged from the cathode.
The exact behaviour of an individual collision is hardly predictable but the number of
possible variations has proven to be limited. When there is little energy in a collision,
the gas atom will temporarily be deformed and tipped out of balance without any
resisting change of its properties. Such so-called elastic collisions will happen when a
gas atom collides with an electron that was not yet able to gain enough speed in the
electric field between the electrodes. In a collision with more energy (or higher speed)
an electron within the atom may get exited and make a transition to a higher band
within its atom. Most of the excited states of an electron are unstable and after a
short while the electron will return to its initial state while emitting a photon with an
electromagnetic radiation that is characteristic for the given type of transition. Certain
types of excited states however, happen to be meta-stable which means that the
electrons are able to stay in their new and higher band for a relatively long period of
time. When such an excited electron gets hit by another free electron it may move
over to an even higher level of energy or it may even escape from its atom, leaving
the atom as a positively charged ion. Ionisation may also occur in a single collision
when the collision speed is sufficiently high.
After the ionisation of an atom by a free electron there are now two free electrons
that each can take speed in the electric field again and can get involved in new
collisions. In a relatively small number of occurrences a free electron may also be
captured by an ion where it then takes over the place of a formerly escaped electron,
again under the release of a photon with a characteristic electromagnetic radiation.
With sufficiently high numbers of involved electrons and atoms all types of collisions
and transitions will occur more or less at the same time. The relative contribution of
the different types of photons to the total amount of electromagnetic radiation is a
statistical property that depends on the environmental conditions. A change in these
conditions like a lower or higher gas pressure or temperature will alter the likeliness
of a certain type of transition but the number of possible transitions will stay the
same. This limitation and the fact that a certain transition produces radiation with its
own characteristic wavelength results in a spectral distribution that is characterised
by more or less clear defined spectral lines in contrast to the more continuous
spectral distribution of a glowing solid.
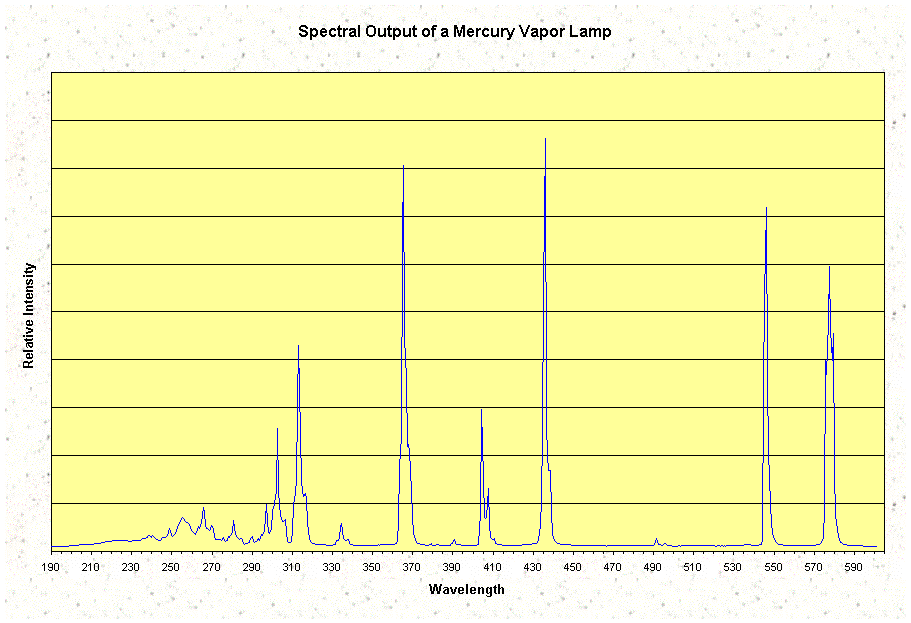
For the construction of a sunlamp it was essential that one or more of the stronger
spectral lines were located within the ultraviolet area. Mercury vapour met this
requirement with energy transitions whose corresponding wavelengths are located at
365 (UV-A) and 313 (UV-A/B) nanometers.