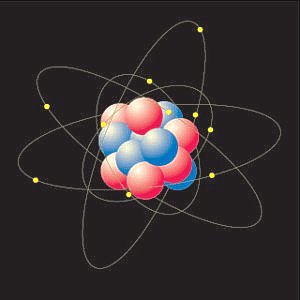
ATOM-DIMENSIONS
The figure next to here depicts an atom on a
non-proportional scale. Non-proportional because a
proton and a neutron are each about 1800 times
heavier than an electron and the distance between
the nucleus and its electrons is much larger than
suggested in the figure. When the nucleus would be
assumed to have the size of a tennis ball, the
distance towards its nearest electron would be
about the height of the Empire State Building. And
you would need in the order of ten billion atoms on a
row to span a distance of one millimeter.