Wilhelm Wien
1864 - 1928
WIENS-DISPLACEMENT-LAW
Like with energy transitions in a gas, the number of
possible vibrational transitions within a solid is
limited too, but the number of possible variations is
far much greater. For this reason the emission
spectrum of a glowing solid lacks the existence of
the well-defined peaks that often can be seen in the
emission spectrum of hot gasses.
A glowing solid is said to have a continuous
emission spectrum although there is a more or less
clear maximum around a certain wavelength. In
1893 Wilhelm Wien discovered that the wavelength
λmax of this maximum is inversely proportional
to the absolute temperature T of the glowing solid.
The relation λmax = b/T is known as Wien's
displacement law. In this formula the temperature T
is given in degrees Kelvin (K). The constant b in the
formula has a value of 2,898 x 10^-3 Kelvin-meter and is called Wien's constant.
Although Wien identified the temperature-depended displacement of the peak in the
emission spectrum of a glowing solid, the formula for the total emission spectrum of a
photon would only be deduced in 1901 by Max Planck.
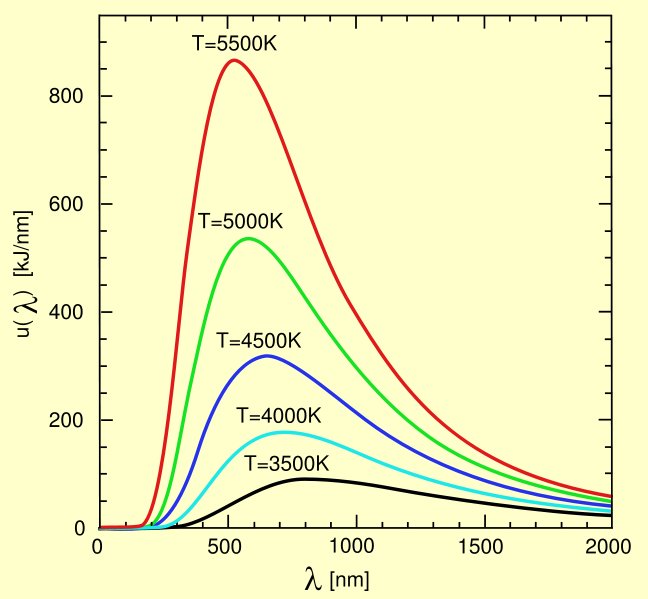
In the figure below, the emission spectrum of a thermally black body is plotted at
different temperatures. A perfect thermally black body absorbs all of the incoming
radiation and does not reflect any of it. The emitted radiation then totally depends on
the temperature of the body itself.
In an electric heat lamp the total amount of radiated energy was proportional to the
electric energy in Joule per second that was added to the device while the
characteristics of its radiation depended on the dimensions of the radiator. More
energy would increase the temperature of the heating element and it made the peak
wavelength shift to the left (the heating element would glow brighter) while a larger
heating element with the same amount of added energy would have a lower
temperature, shifting the peak wavelength to the right. From the figure can be read
that adding power and thus increasing the elements temperature would shift the peak
wavelength of the emission from 800 nm at 3500K (bright lamp light) to 525 nm at
5500K (sunlight). In an ordinary incandescent lamp the temperature stayed limited to
about 2900K with a peak level at about 1000 nm. From the shape of the curve it is
easily to be seen that most of the radiation of an incandescent lamp was infrared
radiation and only a small 10 to 20% was visible light.